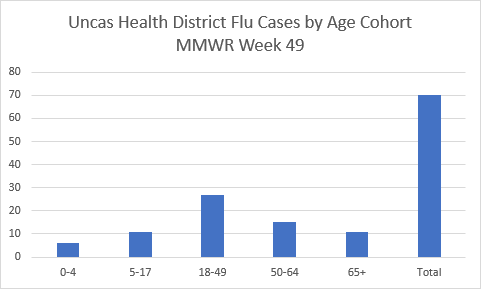
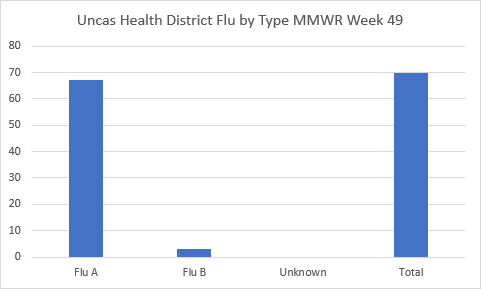
Attention Connecticut parents with children under 5: The clock is ticking on getting your little ones flu-ready! To keep everyone healthy and happy in classrooms this winter, remember that Connecticut immunization regulations require a minimum of one dose of influenza vaccine for children under five each year between August 1st and December 31 to attend Connecticut schools.
Why is this important?
-
Protecting your child: Flu can be especially serious for young children, leading to hospitalization and even death. Vaccination is the best way to shield your little one from the flu.
-
Protecting the community: Children spread germs easily, so vaccinating them helps protect vulnerable individuals at school, like younger siblings or teachers with health conditions.
-
Staying in school: Missing school due to illness can disrupt learning and set kids back. Vaccination means fewer sick days and more fun exploring the world with their classmates.
Getting your child vaccinated is easy!
-
Check with your pediatrician: They can answer any questions you have and administer the vaccine during a regular checkup.
-
Uncas Health District flu clinics: Offered throughout December
-
Other flu clinics: Many health departments and community centers offer free or low-cost flu shots. Find a clinic near you at ct.gov/flu.
-
Remember, the Connecticut Vaccine Program provides vaccines at no cost to all children under 19, regardless of insurance status.
Don’t delay – December 31st is coming fast! Make sure your child is flu-protected and ready for a healthy, happy school year. Let’s all work together to keep our classrooms germ-free and our kids smiling!
Additional tips:
-
Talk to your pediatrician about the specific type of flu vaccine recommended for your child’s age.
-
Schedule your child’s appointment early to avoid a last-minute rush.
-
Remember, even vaccinated children can still get the flu, so continue good hygiene practices like handwashing and staying home when sick.